The thymus is a crucial training ground for T-cells, the body’s “white knights,” where they learn to battle the various diseases they may encounter. Thymic function shrinks to nearly nothing as we age, severely limiting our ability to recognize and defend against cellular infiltrators.
Scientists at the University of Texas Health Science Center at San Antonio (UT Health San Antonio) discovered a crucial pathway in the thymus that determines the rate of growth and functional preservation. Surprisingly, this pathway appears to act through both indirect and direct methods. Understanding these functions could help produce treatments that preserve thymic function for longer, boosting the immune system’s power to fight disease.
A UT Health San Antonio-led study, published in Nature Aging in February 2025, highlights the role of the peptide hormone fibroblast growth factor 21 (FGF21) in regulating T-cells and, potentially, preserving thymic size over time. Principal investigator Ann Griffith, PhD, assistant professor in the Department of Microbiology, Immunology and Molecular Genetics, in the Joe R. and Teresa Lozano Long School of Medicine at UT Health San Antonio, said this research could be pivotal in developing a way to preserve strong immune responses across a lifetime.
Cracking the code of thymic function
The thymus is essential for a healthy immune system, but the causes of age-related thymic atrophy remain a mystery. Scientists have developed approaches for thymic regeneration, but the effects are short-lived. Additionally, while thymic size can be restored, it is unclear if full thymic function is also intact.
Griffith’s study developed from previous work based on a transcriptomic approach investigating every gene expression in the genome and learning how those expressions change as the thymus regenerates. That study revealed that FGF21 and certain signaling pathways corresponded to thymic growth. Regeneration also increased signaling through the mammalian target of rapamycin (mTOR) pathway — a crucial regulator of growth and metabolism. Another discovery was that the size of the individual cells made more of a difference in function preservation than the overall number of cells.
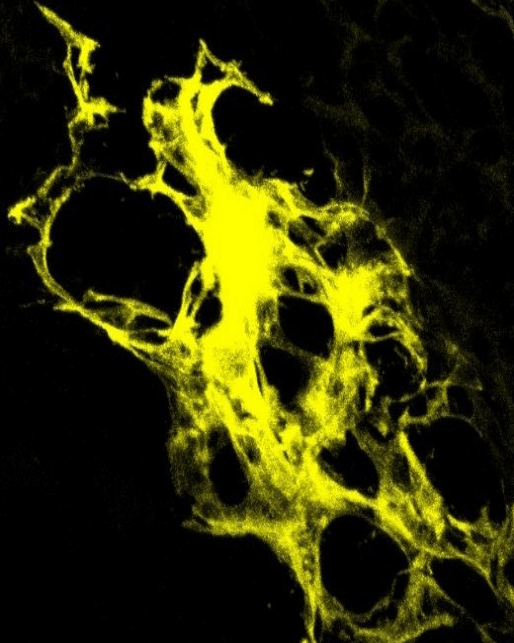
Fighting inflammation, autoimmunity with FGF21
Immature T-cells developing in the thymus are fragile and affected by many kinds of stressors to the body, including cancer treatments like chemotherapy and radiation. Once stressors are removed, the thymus tissue naturally regenerates. However, regrowth is slow, and restoration of young T-cells takes a long time, leaving individuals more susceptible to infections and tumor recurrence.
Training tomorrow’s defenders
Having a variety of T-cells that recognize various infiltrators is important in maintaining and restoring health. As people age and produce fewer newly trained T-cells, the body has trouble fighting novel infections. The total number of T-cells does not diminish with age, but without newly trained recruits, there are merely more cells that recognize the same things.
In mouse model experiments, increasing FGF21 preserved both the size and function of the thymus, allowing for greater T-cell variation.
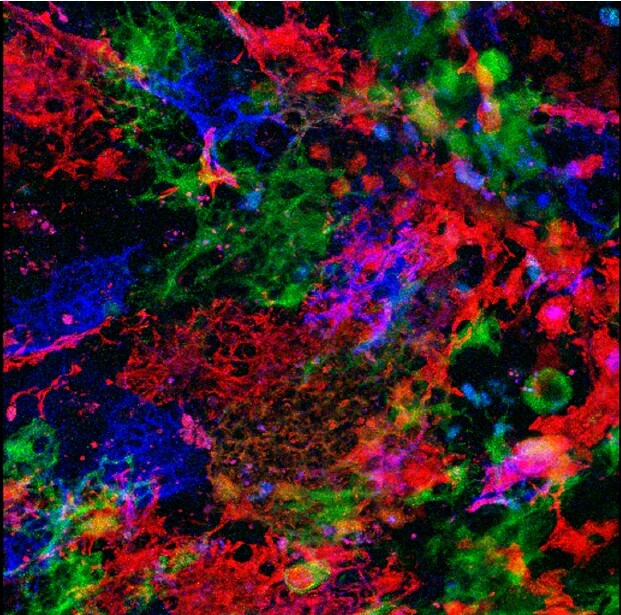
Inside the labyrinth: How thymic structures rebuild
Stromal cells, specifically thymic epithelial cells, where these signals are taking place, have unusual structures, like intricate labyrinths. Each of the tiny mazes can hold about 100 developing T-cells. As the thymus ages, the walls of these structures collapse. Upon thymic regrowth, the walls are rebuilt. Experiments in mouse models showed that an increase in FGF21 could delay thymic atrophy, however, it did not completely prevent it.
“We saw FGF21 impacted the morphology of these labyrinths, and that was associated with overall tissue size. FGF21 can signal directly to the stromal cells inside the thymus and may also be signaling to other cells in a way that impacts the morphology. It’s clear that either directly or indirectly, FGF21 has an impact on the mTOR signaling pathway,” Griffith said.
Restoring immunity for healthier golden years
“Since we are making fewer [new T-cells] as we get older, we have an expansion of the T-cells that have already been exposed to something. We have a lot of memory, but not very much modification. That is one reason why older people do not respond as well to vaccines and are more susceptible to brand-new viruses, like COVID-19. They have a narrower repertoire of pathogens that their T-cells can recognize,” Griffith said.
As T-cell variability shrinks, auto-reactivity to the body’s own cells increases, leading to inflammation. This response was also dampened after an increase in FGF21.
“Part of the T-cells’ education in this ‘school’ is to make sure that if they are recognizing our own tissues, we remove these cells. We’ve seen this function diminish with age and this protein was able to mitigate that to potentially allow for better tolerance in T-cells that come out of the thymus,” Griffith said.
Giffith said this study is an exciting step forward in finding targets that can durably restore thymic function in older people and improve their immune response.
Read more:
Nature Aging News & Views : FGF21 keeps the thymus young
Paracrine FGF21 dynamically modulates mTOR signaling to regulate thymus function across the lifespan
Sarah A. Wedemeyer, Nicholas E. Jones, Iwan G.A. Raza, Freedom M. Green, Yangmig Xiao, Manpreet K. Semwal, Aaron K. Garza, Kahealani S. Archuleta, Kymberly L. Wimberly, Thomas Venables, Georg A. Holländer, Ann. V. Griffith
Nature Aging
First published February 2025.

