Media contacts: Will Sansom, UT Health San Antonio, (210) 567-2579, sansom@uthscsa.edu
Shelley Kofler, University Health, (512) 294-5224, Shelley.Kofler@uhs-sa.com
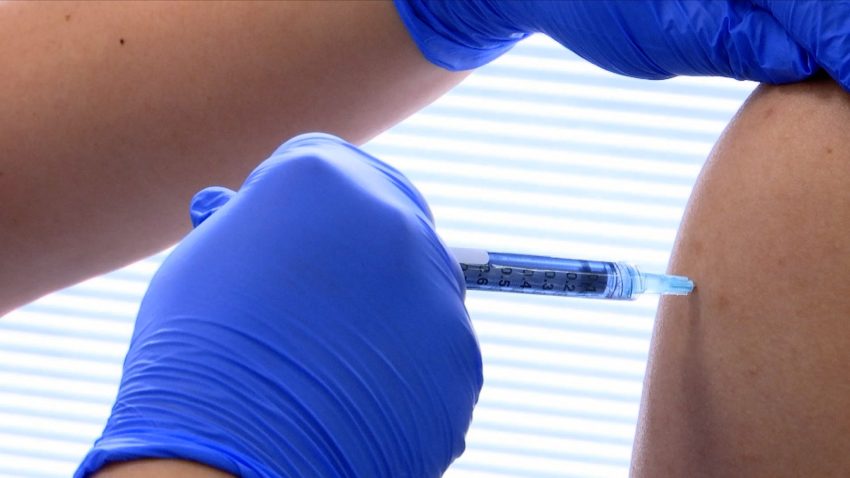
SAN ANTONIO – San Antonians will have the opportunity to participate in the fifth international COVID-19 vaccine clinical trial beginning in January through UT Health San Antonio and its clinical partner, University Health System, now referred to as University Health.
![]()
Barbara Taylor, MD, MS, is principal investigator of the local study site, which is part of the COVID-19 Prevention Network. She is associate professor of infectious diseases at UT Health San Antonio and sees patients through University Health. Dr. Taylor served as chair of the local COVID-19 Health Transition Team last spring and currently serves as co-chair of the COVID-19 Community Response Coalition.
“We are proud to offer this vaccine clinical trial to the people of San Antonio and surrounding counties,” Dr. Taylor said. “Our community has made many sacrifices throughout this pandemic and worked hard on masking and social distancing. We are excited to do our part and work towards a protective vaccine, which would provide another tool to help end the COVID-19 pandemic. We are particularly interested in seeing participation from people at higher risk for COVID-19, including older participants.”
In this Phase 3 randomized control trial, study sponsor Novavax seeks to enroll approximately 30,000 volunteers at up to 110 study sites in the U.S. and internationally. Locally, 500 participants are being sought.
The vaccine candidate, called NVX-CoV2373, is a protein engineered from the genetic sequence of the SARS-CoV-2 virus that causes COVID-19. The vaccine candidate was shown to have a reassuring safety profile and elicited a strong immune response in a Phase I trial in results announced by the company Aug. 4.
Studies completed so far on this vaccine candidate, published in the New England Journal of Medicine Sept. 2, evaluated two injections of the vaccine at two dose levels with and without the company’s patented Matrix-M™ adjuvant, a vaccine ingredient being tested to see if it produces a stronger immune response at a lower vaccine dosage. One hundred thirty-one participants ages 18 through 59 participated in the trial that showed the study vaccine was generally well tolerated. Patients who received the vaccine with Matrix-M had stronger antibody and T-cell responses, both hallmarks of a vaccine that could protect from infection.
The Phase 3 trial will enroll adults age 18 and older across diverse populations, including those at higher risk because of their age, race, ethnicity, medical conditions, living situation or work environment. Participants will randomly receive either the vaccine or placebo in two doses, 21 days apart. Two-thirds of volunteers will receive the vaccine and one-third will receive the placebo. All participants will be followed for two years to determine whether the vaccine reduces the risk of COVID-19 disease. Study participants will be seen at both University Health and UT Health San Antonio locations.
For more information and to volunteer, visit UTHealthResearch.com or call 210-469-3206.
# # #
The University of Texas Health Science Center at San Antonio, also referred to as UT Health San Antonio, is one of the country’s leading health sciences universities and is designated as a Hispanic-Serving Institution by the U.S. Department of Education. With missions of teaching, research, patient care and community engagement, its schools of medicine, nursing, dentistry, health professions and graduate biomedical sciences have graduated more than 37,000 alumni who are leading change, advancing their fields and renewing hope for patients and their families throughout South Texas and the world. To learn about the many ways “We make lives better®,” visit www.uthscsa.edu.
About University Health System, now referred to as University Health
University Health System, now referred to as University Health, is San Antonio’s only locally owned health system and the only academic medical center in South Texas. Its University Hospital serves as the region’s Level I trauma center for adults and children, and is the only area hospital to be state-designated at the highest level for both its Maternity Center and Neonatal Intensive Care Unit. Outpatient care is provided through a comprehensive network of urgent, primary and specialty care centers. For more than 100 years, University Health has been committed to delivering compassionate, culturally competent and high-quality healthcare, based on a strong foundation of outcomes‐based research and innovative teaching. Learn more at UniversityHealthSystem.com. Follow us on Twitter and Facebook.