Researchers at The University of Texas Health Science Center at San Antonio (UT Health San Antonio), reporting this week in the journal Proceedings of the National Academy of Sciences, identified a mechanism through which two antiviral genes, when mutated, promote a childhood cancer called pediatric myelodysplastic syndrome (MDS). Scientists at Oklahoma State University and Cornell University collaborated on the study.
When normal, the genes, called SAMD9 and SAMD9L, suppress tumor formation and help protect against virus infection. They are effective and selective sentinels.

“Normally those two genes are silent in the cells, only becoming activated when they encounter infection,” said senior author Yan Xiang, PhD, professor of microbiology, immunology and molecular genetics in the Joe R. and Teresa Lozano Long School of Medicine. “However, if an individual has a mutation inthese two genes, they are turned on, even without infection. And that can create a lot of diseases.”
Since 2017, studies have found that about 8% of pediatric MDS patients have mutations in the two genes. That makes SAMD9/SAMD9L errors the most common currently known cause of MDS in children. These children have fewer immune cells than normal and a high tendency to develop acute myeloid leukemia.
The new study contributes two key findings:
- Patient-derived SAMD9/SAMD9L mutations cause a stall in protein synthesis in cells and prompt a stress response to abnormal protein synthesis. This could largely explain why children with the mutations have the underdeveloped immune system. Other studies in the last few months have hinted at protein synthesis as the problem.
- SAMD9 and SAMD9L proteins have a specific region that is very critical for their function. When the researchers deactivated this region, they stopped the toxic effects of the mutated proteins, Dr. Xiang said.
The team also found that this protein region performs the toxic function through binding to nucleic acids, which are molecules that store and express genetic information.
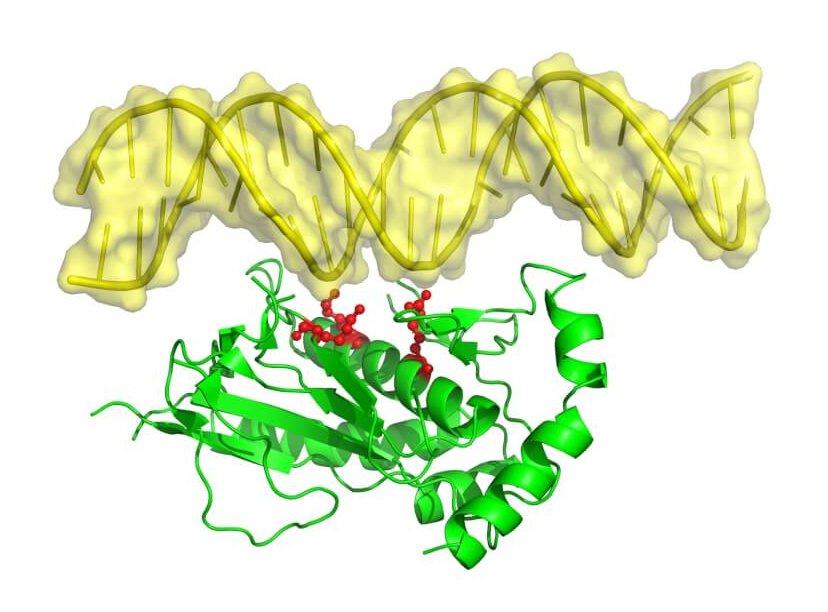
“We obtained a crystal structure of the region to know what it looks like,” Dr. Xiang said, referring to X-ray crystallography studies showing the 3D architecture of the region.
“Since we have the structure of that region, and we know its function, we fully believe we have identified a key therapeutic target for pediatric myelodysplastic syndromes derived from SAMD9 and SAMD9L mutations,” Dr. Xiang said. “We hope to eventually develop a molecule to target that region.”
To date, bone marrow transplant is the only treatment option for children who have SAMD9 and SAMD9L mutations. Families will welcome additional therapies.
MDS is characterized by abnormal stem cell function in the bone marrow, resulting in lower numbers of blood cells than normal. The blood cells are poorly formed and don’t work well. Although rare, MDS risk increases with age.
Pediatric MDS is rare in children. It is diagnosed in approximately 1 in a million babies born each year and represents less than 5% of pediatric hematological malignancies.
Acknowledgments
This work was supported by NIH grant AI151638 (Yan Xiang). Junpeng Deng is also supported by the Oklahoma Agricultural Experiment Station at Oklahoma State University under project OKL03060. Flow cytometry data were generated in the UT Health San Antonio Flow Cytometry Shared Resource Facility (supported by NIH-NCI P30 CA054174 and UL1 TR002645) with help from Sebastian Montagnino. Sequencing data were generated in the UT Health San Antonio Genome Sequencing Facility (supported by NIH-NCI P30 CA054174 and 1S10OD021805-01).
Structure and Function of an Effector Domain in Antiviral Factors and Tumor Suppressors SAMD9 and SAMD9L
Shuxia Peng, Xiangzhi Meng, Fushun Zhang, Prabhat Kumar Pathak, Juhi Chaturvedi, Jaime Coronado, Marisol Morales, Yuanhui Mao, Shu-Bing Qian, Junpeng Deng, Yan Xiang
First published: Jan. 17, 2022, Proceedings of the National Academy of Sciences of the United States of America



